
In 1925, Wolfgang’s Pauli’s atomic model replaced the Bohr model and those based upon it. However, the Sommerfeld model couldn’t accommodate the magnetic quantum number. This allowed the Sommerfeld model to explain atomic effects, such as the Stark effect in spectral line splitting. The Sommerfeld or Bohr-Sommerfeld model significantly improved on the original Bohr model by describing elliptical electron orbits rather than circular orbits. The Bohr model doesn’t explain fine and hyperfine structure of spectral lines.According to the Bohr model, ground state angular momentum is L= ħ. It incorrectly calculates ground state angular momentum.The model violates the Heisenberg Uncertainty Principle because it defines both the radius and orbit of electrons.It doesn’t predict relative intensities of spectral lines.The model couldn’t predict spectra of large atoms.While the Bohr model surpassed earlier models and described absorption and emission spectra, it had some issues: So, the model doesn’t explain why electrons don’t stack in a regular manner. One problem applying the Bohr model to heavier atoms is that the model assumes electron shells don’t interact. The model also explains why noble gases are inert, why atoms on the left side of the periodic table attract electrons, and why elements on the right side (except noble gases) lose electrons. This explains some properties of heavy atoms, such as why atoms get smaller as you move from left to right across a period (row) of the periodic table, even though they contain more protons and electrons. So, the Bohr model for heavier electrons introduces electron shells. When the level filled, additional electrons occupy the next higher level. According to the Bohr model, each orbit only holds a certain number of electrons. Atoms require additional electrons to cancel out the positive charge of multiple protons. The hydrogen atom only contains one proton, while heavier atoms contain more protons. For hydrogen (Z = 1), this line consists of photons with a wavelength of 656 nm (red). The 3 → 2 transition produces the first line of the Balmer series. If an electron moves from one orbit to another, energy is absorbed or emitted. The radius of possible orbits increases as a function of n 2, where n is the principle quantum number. According to the model, electrons only occupy certain orbits. The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. When an electron moves from one orbit to another, energy is absorbed (moving from lower to higher orbit) or emitted (moving from higher to lower orbit).The lowest energy for an electron (most stable state) is in the smallest orbit, which is closest to the nucleus.While gravity determines orbits of planets around stars, electrostatic forces (Coulomb force) causes electrons to orbit the nucleus. Electron orbits are circular, but not all electrons orbit in the same plane (like planets around a star), resulting in spheres or shells where an electron might be found.Electrons have a negative charge and orbit the nucleus.The atomic nucleus consists of protons and neutrons and has a net positive charge.Yet, it’s an important model because it describes the quantum behavior of electrons in simple terms and explains the Rydberg formula for the spectral emission lines of hydrogen. Ultimately, models based entirely on quantum mechanics replaced the Bohr model. Earlier models were the cubic model (1902), plum-pudding model (1904), Saturnian model (1904), and Rutherford model (1911). The Bohr model was the first atomic model incorporating some quantum mechanics. Danish physicist Niels Bohr proposed the model in 1913. It’s called a planetary or cake model because electrons orbit the atomic nucleus like planets orbit the Sun, while the circular electron orbits form shells, like the layers of a cake. The Bohr model or Rutherford-Bohr model of the atom is a cake or planetary model that describes the structure of atoms mainly in terms of quantum theory.
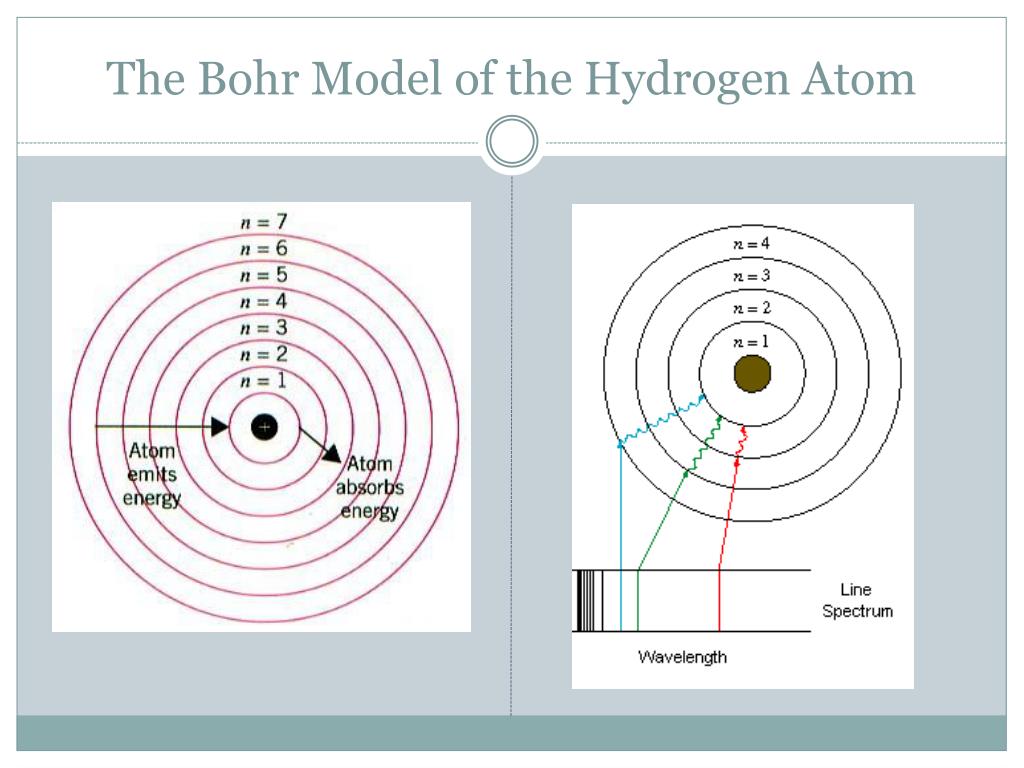
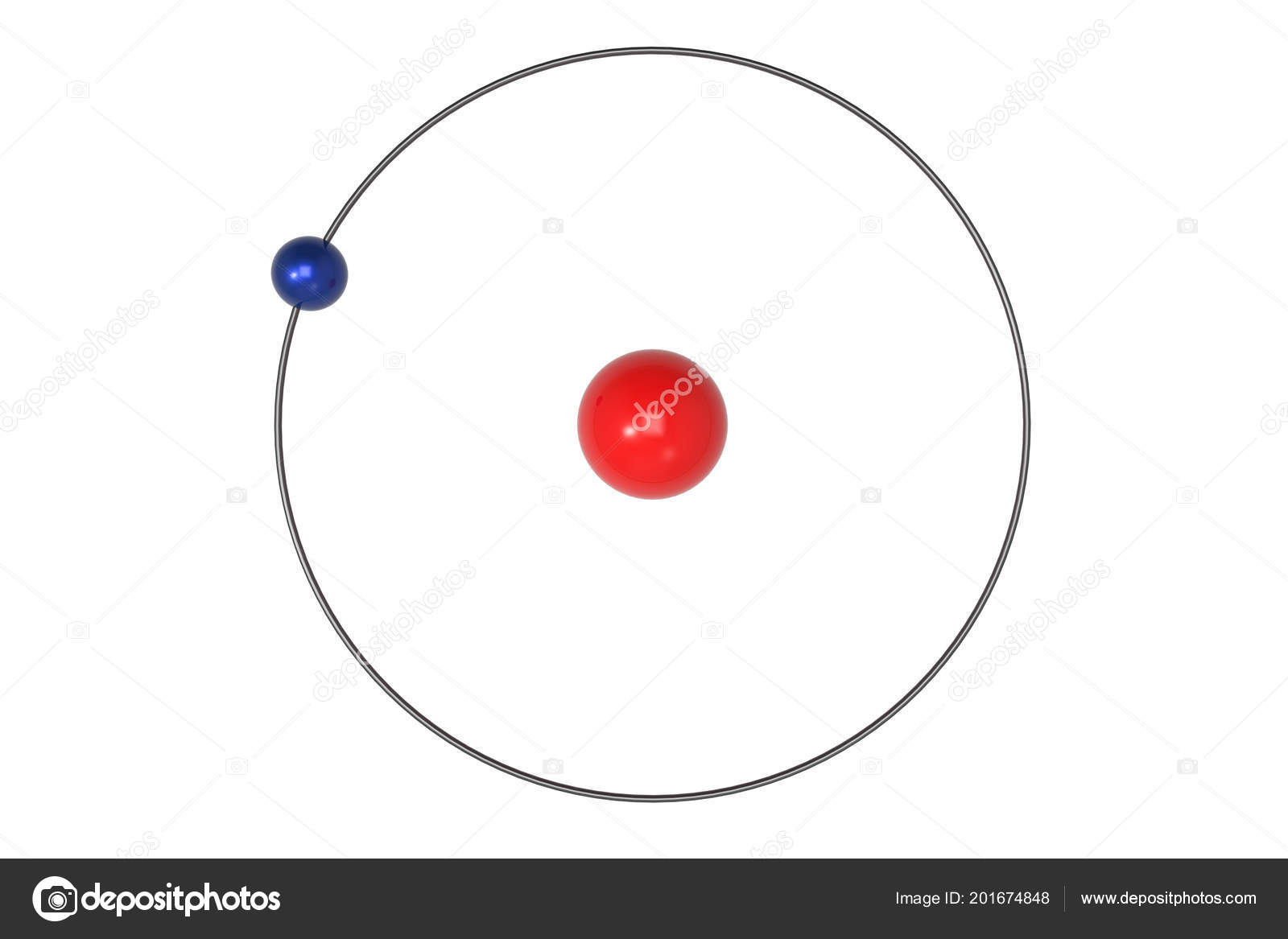
It is the first atomic model based mainly on quantum mechanics. The Bohr model is a cake or planetary model of the atom, with electrons in shells. This entry was posted on Jby Anne Helmenstine (updated on May 10, 2021)


 0 kommentar(er)
0 kommentar(er)
